Photochemical Smog
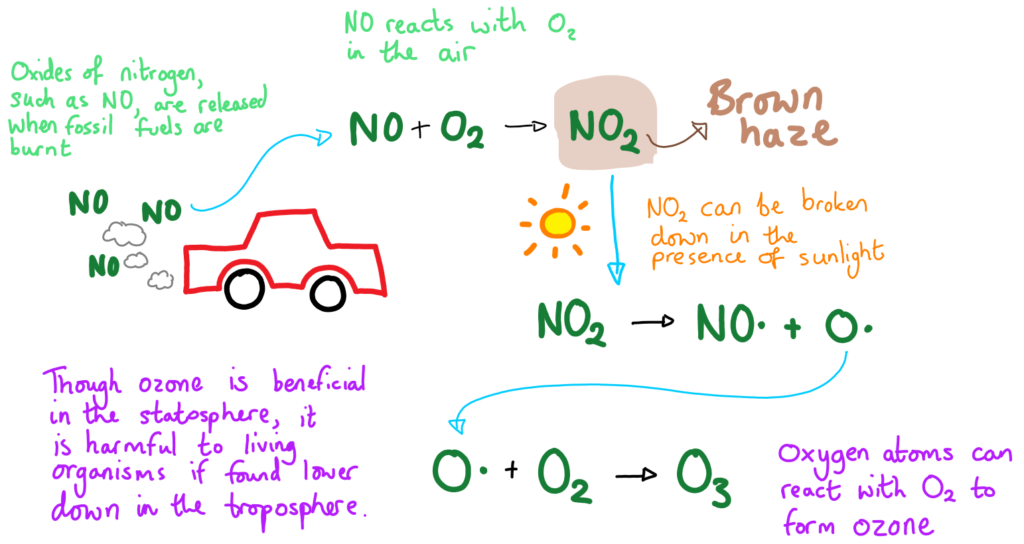
Photochemical smog forms when fossil fuels are burned, releasing pollutants such as oxides of nitrogen (NO and NO₂) and volatile organic compounds (VOCs) into the atmosphere. These oxides of nitrogen contribute to the characteristic brown haze often observed in urban areas.
Under sunlight, these pollutants undergo photochemical reactions (i.e. they react with with the help of energy from the sun), producing ground-level ozone (O₃) and other secondary pollutants. The resulting mixture of ozone, nitrogen oxides, and particulates creates the harmful phenomenon known as photochemical smog, which can cause respiratory problems and environmental harm.
